Abstract
Background : Novel therapeutic approaches for hemophilia A include the bispecific antibody emicizumab (ACE910), for which clinical phase III results were recently reported (Oldenburg et al. NEJM 2017). During preclinical and clinical development, ACE910's activity was tracked using thrombin generation assay (TGA), chromogenic assay, and activated partial thromboplastin time assay (aPTT) (Shima et al. NEJM 2016, Kitazawa et al. Nat Med. 2012). The most suitable method to test the efficacy of non-factor therapy in a routine setting is not yet known.
Aim: To examine the effect of assay type on hemostatic activity monitoring of a sequence identical analogue (SIA) to ACE910, and to assess FVIII-activity equivalence in chromogenic and aPTT assays, and in TGA.
Methods : SIA ACE910 was expressed in HEK293 cells, purified as previously described (Sampei et al. PLoS One. 2013), and analyzed using the following five commercial FVIII chromogenic assay kits: Coatest factor VIII, Coamatic factor VIII, Siemens FVIII:C, Technochrom FVIII:C, and Biophen FVIII:C. aPTT or clot waveform was analyzed using five differently composed trigger reagents (Dapttin, Actin FSL, Pathromtin SL, Triniclot, and APTT-SP). In thrombin generation assays, SIA was tested in comparison with recombinant FVIII (ADVATE) using extrinsic (TF) and intrinsic (FXIa) trigger.Depending on method, SIA concentrations ranged from 2-1200 nM.
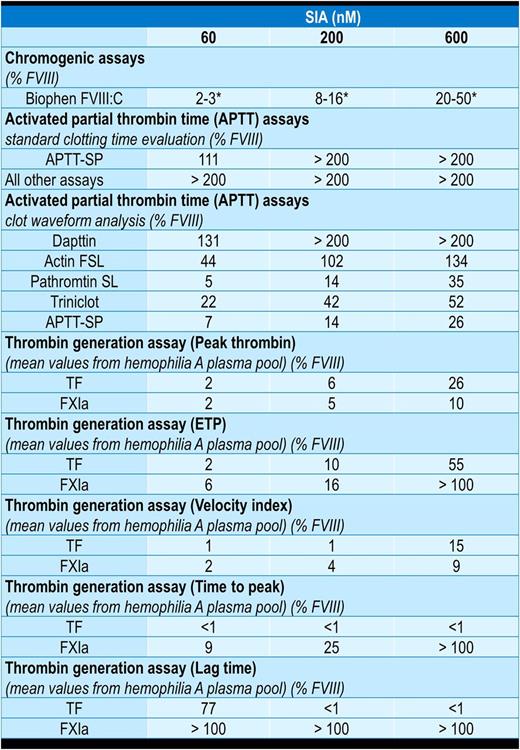
Results: SIA ACE910's lack of cross-reactivity to bovine factors, which are commonly used in chromogenic assay kits, rendered only the Biophen FVIII:C test, which uses human coagulation factors, suitable for analysis. However, as the concentration response curve of SIA ACE910 maxed out and deviated from the FVIII reference curve, the chromogenic assay was not suitable to assess FVIII-equivalence. The aPTT test was highly sensitive to SIA ACE910, resulting in a substantial reduction in clotting time at low and potentially non-therapeutic concentrations. Different aPTT reagents yielded a FVIII-equivalence of 18-69% for 20 nM SIA and >200% at ≥200 nM SIA ACE910 - the plasma concentrations observed in patients in the phase III trial. SIA ACE910 (600 nM) only partially restored thrombin generation (TG) in hemophilia A patient plasma, resulting in a FVIII-equivalence of 4-8% (intrinsic) and 16-36% (extrinsic) based on peak thrombin. Assessment of other TG parameters demonstrated in FVIII-equivalence of <1 to >100% . Assay results for 60, 200, and 600 nM SIA are summarized in Table 1.
Conclusions : Analysis of SIA and its equivalence to FVIII is challenged by its strongly differing mechanism, rendering standard FVIII protocols unsuitable.Results are highly influenced by assay type, analytical conditions, and parameters used for calculation. Chromogenic assays, aPTT, and TGA give largely discrepant values. Thus, new methods and likely a product specific standard are required to better predict the hemostatic effect of non-factor therapies.
Hartmann: Shire: Employment. Feenstra: Shire: Employment. Knappe: Shire: Employment. Schrenk: Shire: Employment. Scheiflinger: Shire: Employment, Equity Ownership; Baxter: Equity Ownership. Dockal: Baxter: Equity Ownership, Patents & Royalties; Shire: Employment, Equity Ownership; Baxalta: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal